 NEW
NEW
What is Hard X-ray Photoelectron Spectroscopy?
2015.09.02 UpdateTOPICS
What is Hard X-ray Photoelectron Spectroscopy?
Background
Photoelectron spectroscopy is an analysis method first reported by Kai. M. Siegbahn in the 1950s. At that time, his report was about photoelectron spectrum for Cu 1s using the MoKα x-ray source 1). Later, there were reports on photoelectron Spectroscopy using hard x-ray in the 1970s 2), but due to technical issues such as lack of strength in the excitation source hard x-ray and lack of photoelectron collection capability in the analyzer, it never reached the level of an analysis method suitable for practical use. Thus, practical application of photoelectron spectroscopy was achieved using soft x-rays such as AlKα and MgKα x-ray source, and ultraviolet sources such as helium discharge tube, and this technology is broadly used today as a powerful analysis method.
Moving into the 21st century, with the emergence of third-generation synchrotron radiation facilities represented by SPring-8, hard x-ray with dramatically increased strength has become available. At the same time, the pressure resistance and sensitivity performance of the analyzer have improved, resolving the technical issues mentioned earlier. As a result, the number of reported cases of hard x-ray Photoelectron Spectroscopy3)4)5) has increased rapidly.
The number of reported cases of hard x-ray photoelectron spectroscopy is still increasing today, attracting attention as a relatively new analysis method.

Photograph of SPring-8 facility
The basic principle of Hard X-ray Photoelectron Spectroscopy
The basic principle of hard x-ray photoelectron spectroscopy is similar to that of general XPS, which irradiates excitation light on the sample surface and measures the kinetic energy of the photoelectrons emitted. While the photon energy of the monochromatic AlKα x-ray source most commonly used in traditional XPS instruments is 1486.6 eV, the photon energy of the excitation source used in hard x-ray photoelectron spectroscopy is 5 to 8 keV, more than triple. Therefore, the photoelectron spectrum achieved using hard x-ray contains a lot of information that was not available with general XPS.
“Hard x-ray photoelectron spectroscopy” is abbreviated as HX-PES or HAXPES. In order to differentiate it from this, photoelectron spectroscopy using soft x-rays such as AlKα and MgKα x-ray source as the excitation source is sometimes called “Soft X-ray photoelectron spectroscopy” (abbreviation: SX-PES).
Advantages of Hard X-ray Photoelectron Spectroscopy
Information from Deep Core Level
Due to the high incident energy of hard x-ray, photoelectrons from a deeper core level can be excited compared to soft x-ray. For example, when the target is Si, soft x-ray can excite up to the 2s orbit only, but hard x-ray can excite up to the 1s orbit. Figure 1 shows an example of photoelectron excitation using AlKα x-ray source (hν=1486.6 eV) and CrKα x-ray source (hν=5414.8 eV).
Figure 1. Diagram of photoelectron excitation on Si
While photoelectron excitation from the core is possible, when the excitation energy becomes high, photoionization cross-section decreases significantly. Photoionization cross-section is a physical quantity associated with the process of photoelectron excitation, and the strength of the spectrum is proportional to this value. Figure 2 plots the photoionization cross-section of Si and Ag by excitation energy. In the Si 2p3/2 and Ag 3d5/2 mainly measured with AlKα x-ray source, when the excitation energy increases from 1.5 keV to 5.0 keV, photoionization cross-section decreases by one to two digits. However, with Si 1s and Ag 2p3/2, which can be measured with CrKα X-ray source, the decrease in photoionization cross-section associated with excitation energy is small, and the ionization cross-section at excitation energy 1.5 keV and 5.0 keV are more or less the same.
Thus, since hard x-ray photoelectron spectroscopy can measure more core levels compared to soft x-ray, the most appropriate level can be measured depending on the objective by avoiding overlapped peaks or selecting deep core level.
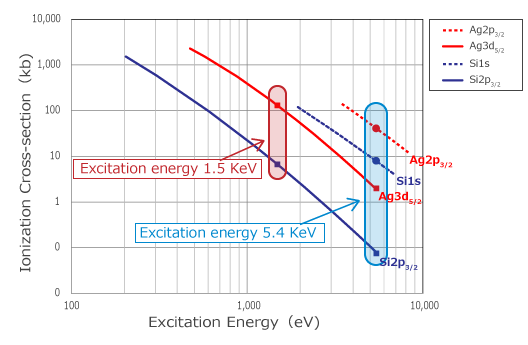
Figure 2. Photoionization cross-section for excitation energy
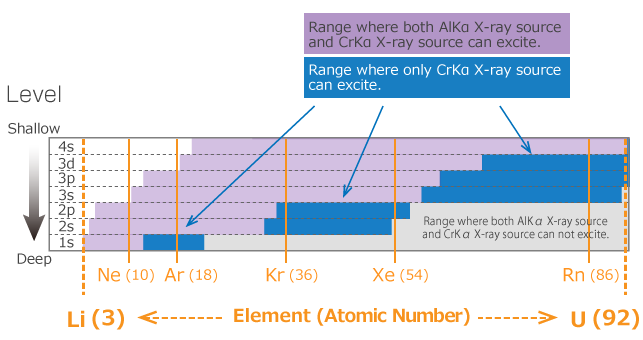
Figure 3. Elements and energy level that can be excited by each X-ray source
Information from Deep Areas
The escape depth of a photoelectron is similar to the Inelastic Mean Free Path (IMFP) of an electron in materials, and the depth of the photoelectron information received as a signal is known to be around two to three times the IMFP. An advantage of hard X-ray photoelectron spectroscopy is that information from deeper areas can be obtained by using high-energy X-ray.
For example, in the case of AlKα and CrKα X-ray source, CrKα X-ray source can obtain information from a depth three times deeper than that of AlKα X-ray source. When measuring with CrKα X-ray source, the impact of contamination, absorbed species, and natural oxidation layer become relatively smaller, so a photoelectron spectrum closer to the original sample information can be obtained. Additionally, nondestructive analysis of the surface where the sample is buried is possible, raising expectations for samples that were difficult to evaluate with AlKα X-ray source.
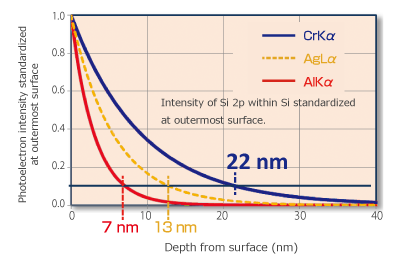
Figure 4. Difference in information depth between CrKα and AlKα X-ray source



